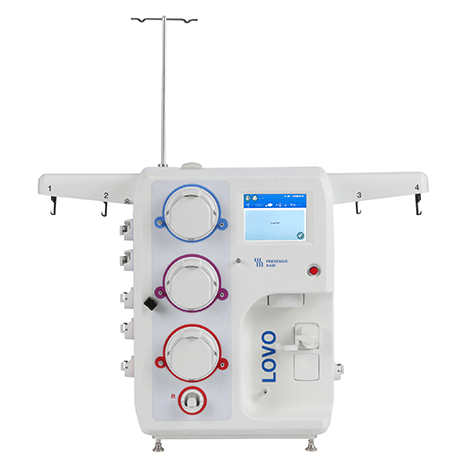
Lovo Cell Processing System
Product # 6R4900
Automated, functionally closed cell processing system with spinning membrane filtration.

CompoSeal Mobilea II
Product # 9027011
Battery operated, small, lightweight, radio frequency (RF) sealer for medical PVC tubing. Includes Handheld sealer, Powerpack, Charger, and Transport Case.

LOVO Cell Processing Disposable Kit with Bag Access (A)
Product # X6R4909A
Single Use Disposable Kit for the Processing of Cells. Compatible with Lovo software versions 3.0 and later
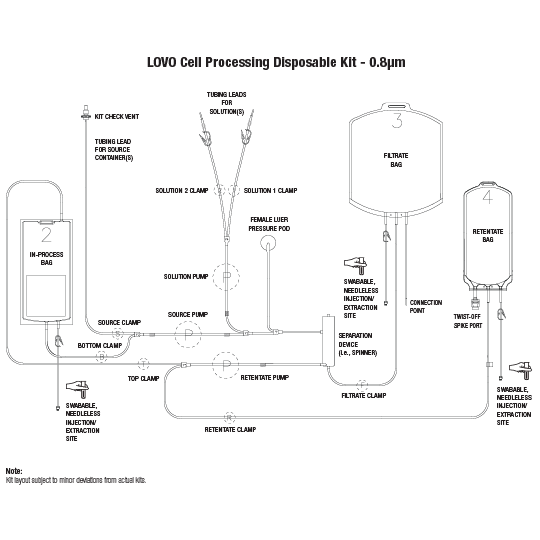
LOVO Cell Processing Disposable Kit - 0.8µm
Product # X6R4930
Single Use Disposable Kit for the Processing of Cells. Compatible with Lovo software versions 3.0 and later

Lovo Ancillary Bag Kit
Product # X6R4902
The Lovo Ancillary Bag Kit is a single unit comprising of a 2.5 L bag with injection site, male luer with non-vented cap, and female luer with vented cap.
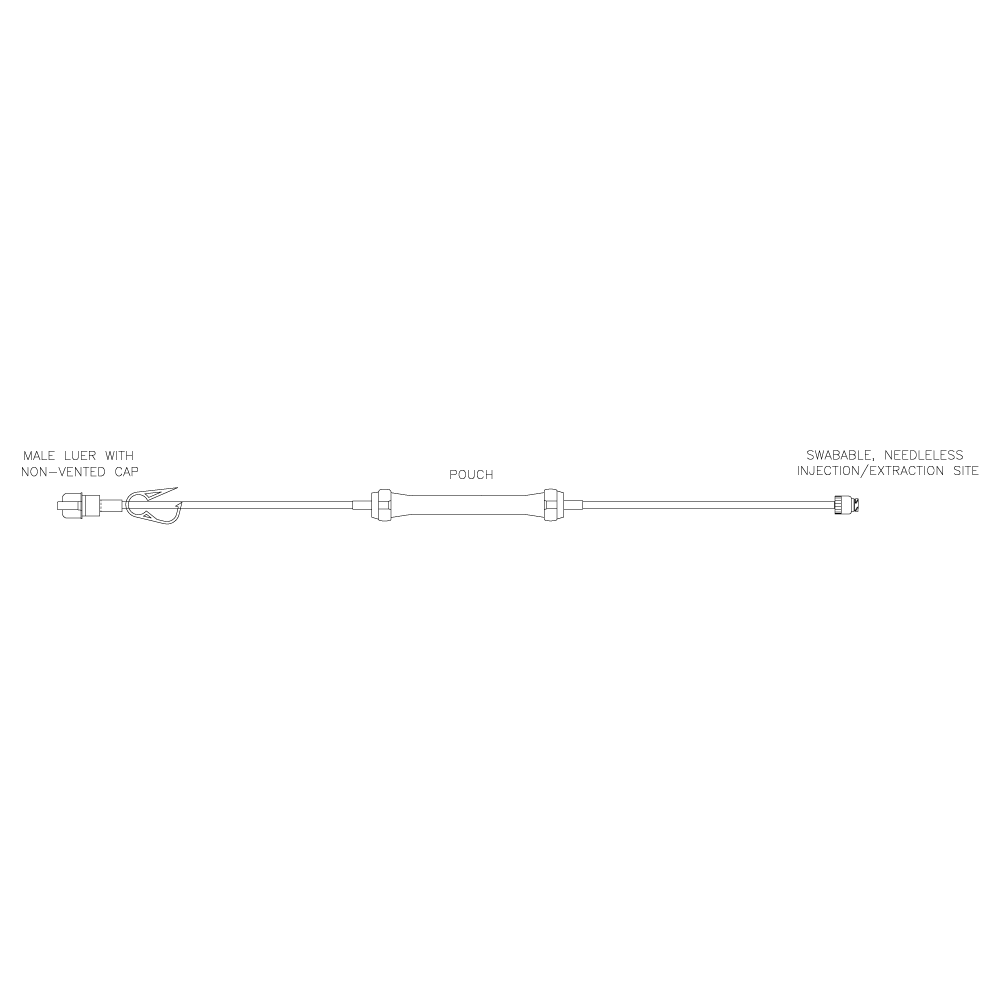
Lovo Pouch Kit
Product # X6R4907
Includes a sterile fluid path and is comprised of an approximately 8 mL pouch component with a swabable, needleless injection/extraction site and a male Luer with a non-vented cap. Single use kit for reagent addition and/or sampling during cell processing.
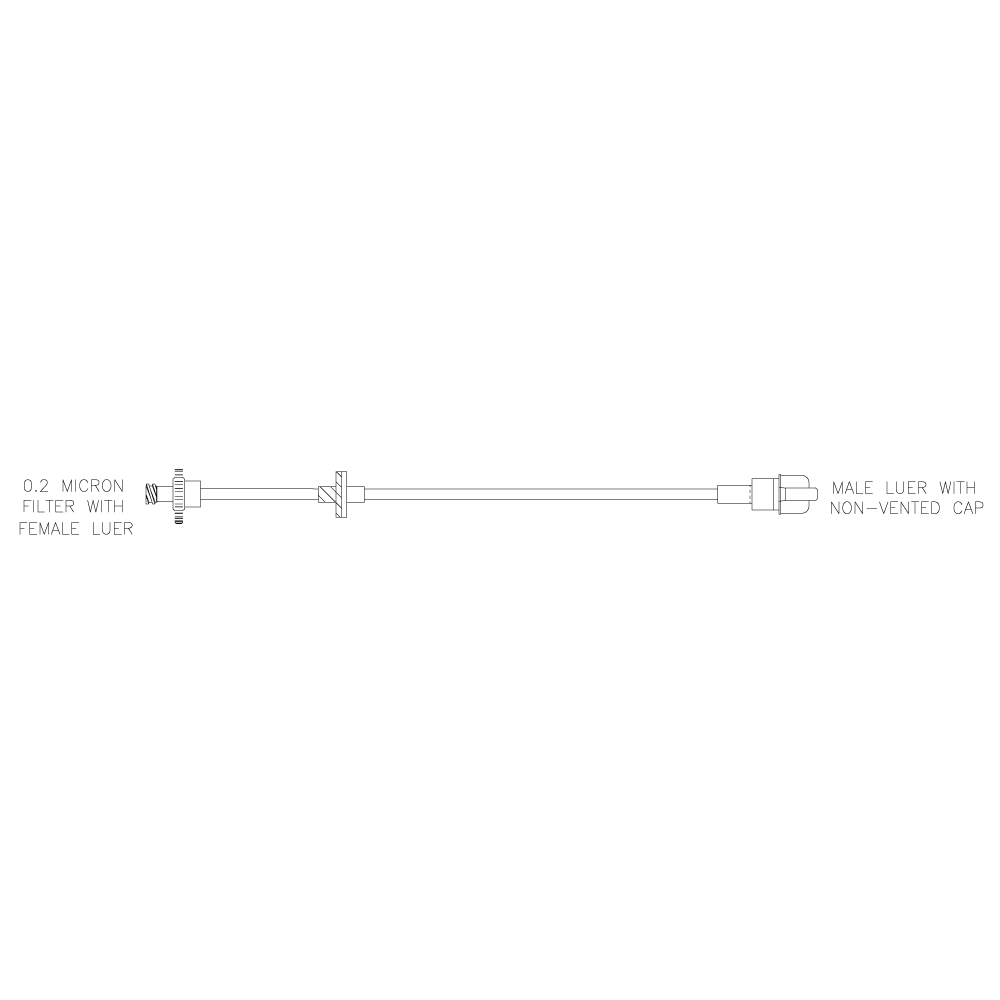
Lovo Valve and Filter Kit
Product # X6R4917
Includes a sterile fluid path and is comprised of a 0.2 μm filter with a female Luer, a check valve, and a male Luer with a non-vented cap. Single use kit for reagent addition and/or sampling during cell processing.
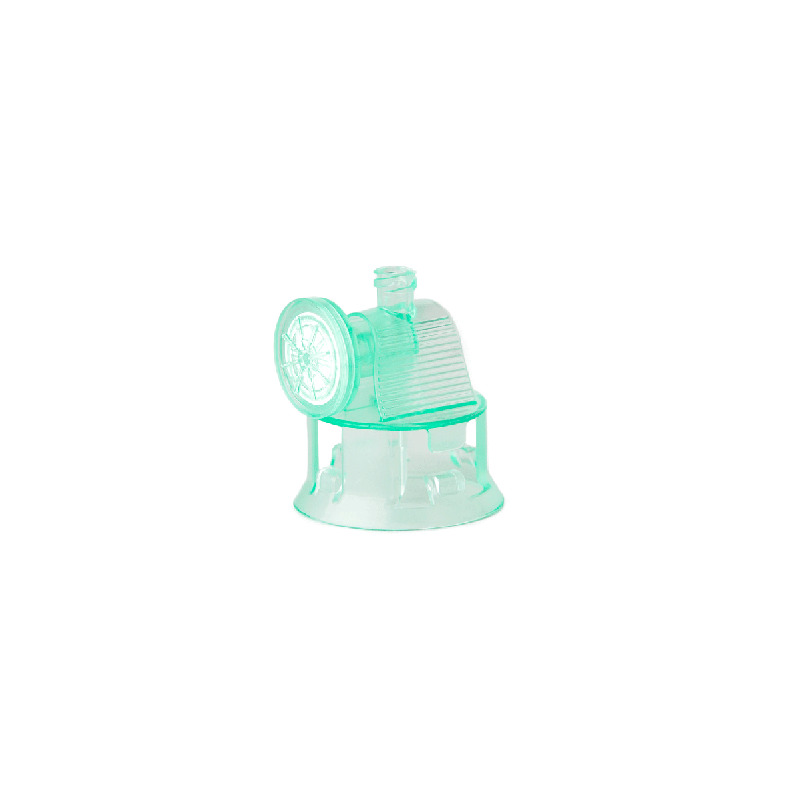
Vialok Vented Vial Access Device
Product # YM020
Yukon Medical’s ViaLok Vented Vial Access device is used to access standard drug vials for needle-free drug preparation and administration.
ViaLok Vented Vial Access Devices have been cleared for sale in the U.S. and E.U. only.
Certificates of Compliance
Download ISO certificates and access certificates of sterility, analysis or origin based on product code or number.
Fresenius Kabi Catalog
Access our complete catalog of medical devices, specialty products and accessories.
The Lovo Cell Processing System is for laboratory use only and may not be used for direct transfusion. Appropriate regulatory clearance is required by the user for clinical use.
For applications requiring regulatory clearance or approval, Users may request required Lovo technical documentation from Fresenius Kabi to support their submission.
Refer to the Lovo Cell Processing System Operator’s Manual for a complete list of warnings and precautions associated with the use these products.
Trademarks referred to are property of their respective owners.